Novel organic semiconductors with specific substitution pattern – Publication by A2 (Witte) & A8 (Koert)
In their study published in Angewandte Chemie International Edition, the authors from two SFB-projects present their research into unilaterally fluorine-substituted pentacenes. This new reaction path will make it possible to synthesize functional materials and to create molecular nanostructures, the properties of which can be used in future organic components.
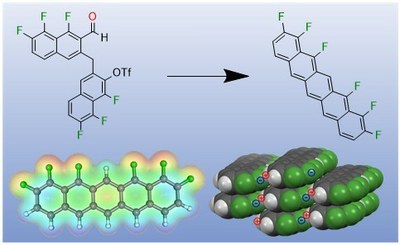
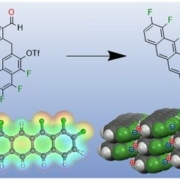
A ring closing reaction leads to a new unilaterally fluorine-substituted pentacene with a novel packing motif in the solid. This product exhibits a strong dipole moment (picture: Daniel Bischof).
Organic semiconductor materials consist of polycyclic aromatic hydrocarbons (PAH), which serve as molecular building blocks for the realization of functional materials and thin-film devices. The electronic properties of these often structurally simple materials can be changed by specific chemical modifications and thus tailored to the respective application. For example, perfluorination of such p-conjugated molecular materials affects the polarity of the charge carrier thus allowing a change from p-type to n-type semiconductors. For the prototypical organic semiconductors of acenes, it has so far only been possible to implement substitution patterns in which the molecules have been substituted symmetrically with respect to their long axis or entire ring units have been modified. In a recent collaboration between synthetic chemistry and molecular solid-state physics at the Philipps-University in Marburg, a novel synthetic strategy has been introduced that enables a regioselective functionalization of acenes.
This new concept was demonstrated using the example of unilaterally substituted fluoroacenes, whose electronic structure is a hybrid of the parental non-fluorinated and perfluorinated pentacenes. An important milestone in the synthesis strategy developed in the group of Prof. Dr. Ulrich Koert is the transition metal-catalyzed C-C bond formation, which makes this synthesis controllable. This novel material was crystallized and characterized with respect to its optical and electronic molecular- and solid state properties in the group of Prof. Dr. Gregor Witte. It was shown that the unilateral fluorination causes a distinctive dipole moment in contrast to the symmetrical substituted molecules. In addition, the molecules in crystals show a novel packing motif and also their optical solid states (excitons) are significantly altered. The identified new molecular packing motif and the additional electrostatic interactions open up new possibilities for the controlled fabrication of functional thin films and molecular heterostructures with special molecular interface structures and thus enable a tailoring of the electronic interface coupling.
Publication
P.E. Hofmann, M.W. Tripp, D. Bischof, Y. Grell, A.L.C. Schiller, T. Breuer, S.I. Ivlev, G. Witte, U. Koert,
Unilaterally Fluorinated Acenes: Synthesis and Solid State Properties,
Angewandte Chemie 59, 16501 (2020)
Contact
Prof. Dr. Ulrich Koert
Philipps-Universität Marburg
SFB 1083 project A8
Tel.: +49 6421 28-26970
E-mail: koert@chemie.uni-marburg.de
Prof. Dr. Gregor Witte
Philipps-Universität Marburg
SFB 1083 project A2
Tel.: +49 6421 28-21384
E-Mail: gregor.witte@physik.uni-marburg.de